
Ken Witt, PhD
Professor of Pharmaceutical Sciences
Pharmacology
B.S., 1994, Chemistry, Northern Michigan University
Ph.D., 2004, Pharmacology and Toxicology, University of Arizona
Postdoctoral, 2001-2005, University of Arizona
Email: [email protected]
Biography
Dr. Witt received a BS in chemistry from Northern Michigan University and a PhD in pharmacology and toxicology from the University of Arizona, College of Medicine. Dr. Witt did postdoctoral work in the Department of Medical Pharmacology also at the University of Arizona, College of Medicine. He joined the faculty of the School of Pharmacy, Southern Illinois University Edwardsville (SIUE) in 2005.
Professional Positions
| 1994 - 1997 | Environmental Chemist, Tri-Matric Laboratories, Grand Rapids, MI |
| 2005 - 2011 | Assistant Professor, School of Pharmacy, SIUE |
| 2005 - 2015 | Research Scientists, (WOC) Veterans Affairs Medical Center, St. Louis, MO |
| 2011 - 2017 | Associate Professor, School of Pharmacy, SIUE |
| 2010 - present | Adjunct Professor, Chemistry, SIUE |
| 2017 - present | Professor, School of Pharmacy, SIUE |
| 2018 - present | Adjunct Professor, Pharmacology-School of Medicine, Southern Illinois University |
| 2018 - present | CEO/Founder Somatolynk Inc. |
Research
Research focused in neuropharmacology, corresponding with drug development, pathological outcomes, and blood-brain barrier (BBB) influences. My laboratory utilizes numerous analytical and molecular biology technologies, with a strong history with rodent modeling. Two primary areas of current research are in Alzheimer’s disease drug discovery/development and BBB pathology:
1. Primary drug discovery/development research focuses on the advancement of an orally bioavailable somatostatin receptor subtype-4 (SSTR4) selective drug candidate with disease-modifying attributes for the treatment of Alzheimer’s disease (AD). AD is the leading cause of dementia that results from the destruction of the brain’s neuronal networks. It is estimated that 1-in-8 individuals over the age of 65 has AD, with a prevalence of 1-in-3 over age 85. There are ~5.8 million people in the USA
with AD, which is projected to increase to 13.8 million by 2050. (Alzheimer's disease facts and figures 2019). SSTR4-AD video: https://www.youtube.com/watch?v=UMs39IvFG_M
The AD drug development pipeline to date has heavily focused on amyloid-beta (Aβ) antibodies and enzymatic inhibitors of Aβ generation. Both antibodies and enzyme inhibitors have seen repeated failures in meeting clinical endpoints due to toxicity (secretase inhibitors) or failure to get adequately into the brain (antibodies). Many of the “newer” antibodies and enzyme inhibitors are simply redesigns going after the same targets, and thus focus on the same failed pharmacological approach. **Whereas, SSTR4 represents a novel and highly viable central nervous system (CNS) target, with a different approach and position within the market. Advantages include: 1) CNS GPCR therapeutic targeting with small molecules has a long history of success across numerous diseases/conditions, with established means of optimization (i.e. druggable). 2) SSTR4 is heavily localized to key brain regions affected by AD, with limited peripheral distribution (i.e. brain targeted). This reduces likelihood of side-effects. 3) Mechanism of action centers on enzymatic clearance of Aβ-oligomers via downstream enhanced neprilysin (enzyme) activity. Such Aβ-oligomers contribute to early AD development and are more neurotoxic then plaques. Aβ-oligomers accumulate early in AD-patients and AD animal models resulting in impaired learning and memory, neurodegeneration, tau pathology, inflammation and oxidative stress, and downstream plaques. 4) Neprilysin enzymatic activity downstream of SSTR4 agonist actions in the brain should also mitigate any possible “on/off” effects. This reduces potential side-effects. 5) Added benefit capability via direct cognitive enhancement, which may help beyond early AD stages. 6) Oral delivery focus. Oral administration is best for ease of use and patient compliance over extended treatments. A significant advantage over biologics. 7) Such an approach would represent a first-in-class AD drug.
2) Another area of research interest is in the field of blood-brain barrier (BBB) alterations with pro-inflammatory high-fat “Western” diets. The BBB represents the principle interface between peripheral and central nervous system. Obesity associated with increased peripheral inflammation and numerous co-morbidities holds significant ramifications respective to BBB integrity and CNS disease outcomes. Alterations in diet/metabolism shown to mitigate BBB tight junction protein regulation and integrity may have broad reaching implications. Correspondingly, the potential benefit of key omega-3 polyunsaturated fatty acids (PUFAs) on BBB tight junctions remains to be fully investigated. There are also pharmacological connections, respective to peroxisome proliferator-activated receptor-γ activation and dietary fat interactions, which hold a good deal of interest to the research and health-care community.
Contributions to Science
Drug discovery and Lead Optimization
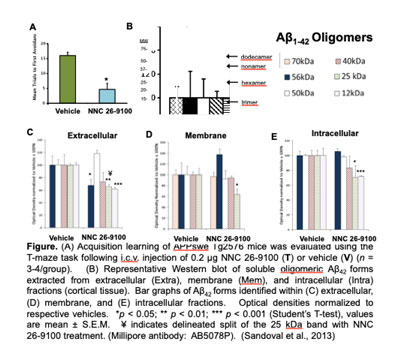 Our Alzheimer’s disease (AD) studies identify SSTR4 agonist actions enhance learning and memory (T-maze, Object Recognition), with disease-modifying actions in pre-clinical AD mouse models (APPswe, 3xTg, and SAMP8) across i.c.v., i.p. and oral administration. SSTR4 agonist (NNC 26-9100) administration (i.c.v.) increased learning and decreased protein expression levels of toxic Aβ42 oligomers (Figure). APPSWE-Tg2576 mice treated with SSTR4 agonist had a decrease in the 56*kDa (dodecamer), 25kDa (hexamer), and 12kDa (trimer) Aβ42 oligomeric bands, within the extracellular cortical fraction. These toxic oligomers represent specific Aβ42 forms involved in loss of cognition. We further evaluated SSTR4 agonist NNC 26-9100 effects on brain mRNA expression against key genes associated with AD pathology in 3xTg-AD mice. SSTR4 agonist treatment further increased activity of Aβ degrading enzyme neprilysin, along with cortical tissue mRNA expression of Sstr4 (4.9-fold), neprilysin (9.3-fold) and insulin degrading enzyme (14.8-fold). This reaffirms SSTR4 sustainability after agonist treatment, and supports the enzymatic mechanism. Preclinical data strongly support SSTR4 agonist actions mitigate AD pathology.
Our Alzheimer’s disease (AD) studies identify SSTR4 agonist actions enhance learning and memory (T-maze, Object Recognition), with disease-modifying actions in pre-clinical AD mouse models (APPswe, 3xTg, and SAMP8) across i.c.v., i.p. and oral administration. SSTR4 agonist (NNC 26-9100) administration (i.c.v.) increased learning and decreased protein expression levels of toxic Aβ42 oligomers (Figure). APPSWE-Tg2576 mice treated with SSTR4 agonist had a decrease in the 56*kDa (dodecamer), 25kDa (hexamer), and 12kDa (trimer) Aβ42 oligomeric bands, within the extracellular cortical fraction. These toxic oligomers represent specific Aβ42 forms involved in loss of cognition. We further evaluated SSTR4 agonist NNC 26-9100 effects on brain mRNA expression against key genes associated with AD pathology in 3xTg-AD mice. SSTR4 agonist treatment further increased activity of Aβ degrading enzyme neprilysin, along with cortical tissue mRNA expression of Sstr4 (4.9-fold), neprilysin (9.3-fold) and insulin degrading enzyme (14.8-fold). This reaffirms SSTR4 sustainability after agonist treatment, and supports the enzymatic mechanism. Preclinical data strongly support SSTR4 agonist actions mitigate AD pathology.
 Our drug development program has led to the discovery of a 3,4,5-trisubstituted-1,2,4-triazole series. Our team synthesized >150 non-peptide SSTR4 focused compounds (NMR validated, >99% pure) with the triazole scaffold, with various modifications of linker chains performed. SSTR4 ligand binding affinities (human SSTR4) of all synthesized compounds to date have been conducted, with appropriate primary and secondary biologic screens. Several of the 1,2,4-triazoles fell into the “viable” range for further advancement, including several compounds with sub-nM SSTR4 binding (Ki) affinity (~1-log unit greater affinity than the endogenous ligand - somatostatin). These compounds showed clear selectivity for SSTR4, on average showing a >500-fold selectivity for SSTR4 over other SSTR subtypes, and selective across 46 other GPCRs.
Our drug development program has led to the discovery of a 3,4,5-trisubstituted-1,2,4-triazole series. Our team synthesized >150 non-peptide SSTR4 focused compounds (NMR validated, >99% pure) with the triazole scaffold, with various modifications of linker chains performed. SSTR4 ligand binding affinities (human SSTR4) of all synthesized compounds to date have been conducted, with appropriate primary and secondary biologic screens. Several of the 1,2,4-triazoles fell into the “viable” range for further advancement, including several compounds with sub-nM SSTR4 binding (Ki) affinity (~1-log unit greater affinity than the endogenous ligand - somatostatin). These compounds showed clear selectivity for SSTR4, on average showing a >500-fold selectivity for SSTR4 over other SSTR subtypes, and selective across 46 other GPCRs.
- Neumann W, Sandoval K, Mobayen S, Minaeian M, Kukielski S, Srabony K, Frare R, Slater O, Farr S, Niehoff M, Hospital A, Kontoyianni M, Crider A, Witt K (2021) Synthesis and structure–activity relationships of 3,4,5-trisubstituted-1,2,4-triazoles: high affinity and selective somatostatin receptor-4 agonists for Alzheimer's disease treatment. RSC Medicinal Chemistry, 2021, 12, 1352 – 1365.
- Schober J, Polina J, Walters F, Scott N, Lodholz E, Crider A, Sandoval K, Witt K (2021) NNC 26-9100 increases Abeta1-42 phagocytosis, inhibits nitric oxide production and decreases calcium in BV2 microglia cells. PloS one 16:e0254242.
- Sandoval K.E., Umbaugh D.S., Crider, A.M., and Witt, K.A., Alterations in brain mRNA expression of beta amyloid degrading enzymes and somatostatin receptor subtype 4 with NNC 26-9100 treatment in 3xTg mice Neurochem Res. 2019 Oct 19.
- Witt K. Crider A, Neumann W., Hospital A., Sandoval K., Kontoyianni M. (2018) 1,4,5-Trisubstituted-1,2,4-triazoles and 3,4,5-Trisubstituted-3-Thio-1,2,4-triazoles and Uses Thereof. In: #PCT/US2018/032368 FaIPA (ed).
- Daryaei I., Sandoval K., Witt K., Kontoyianni M., Crider A. Discovery of 3,4,5-trisubstituted-1,2,4-Triazole agonist with high affinity and selectivity at the somatostatin subtype-4 (sst4) receptor. Med Chem Communications, 9, 2083, 2018..
- Farr S.A., Sandoval K.E., Niehoff M.L., Witt K.A., Kumar V.B., Morley J.E. Peripheral Administration of GSK-3β Antisense Oligonucleotide Improves Learning and Memory in SAMP8 and Tg2576 Mouse Models of Alzheimer’s Disease, Journal of Alzheimer’s Disease Oct 18:54(4): 1339-1348, 2016.
- Sandoval KE, Witt KA, Crider AM, Kontoyianni M (2014) Somatostatin Receptor-4 Agonists as Candidates for Treatment of Alzheimer’s Disease. In: Atta-ur-Rahman, Choudhary MI (eds) Frontiers in Drug Design and Discovery. Bentham Science, (6) Ch12, 566-597.
- Sandoval K.E., Farr S.A., Banks W.A., Crider A.M., Morely J.E., and Witt K.A. Somatostatin receptor subtype-4 agonist NNC 26-9100 mitigates the effect of soluble Aβ42 oligomers via a metalloproteinase-dependent mechanism, Brain Research, 2013, Jul3, 1520: 145-156.
- Wang X., Mealer D., Rodgers L., Sandoval K, Witt K., Stidsen C., Ankersen M., Crider A.M.. Synthesis of 2-Thiohydantoins as Somatostatin Receptor Ligands, Letters in Drug Design and Discovery, 2012, 9 (7):655-662.
- Sandoval K.E., Farr S.A., Banks W.A., Crider A.M., Morely J.E., and Witt K.A. Somatostatin Receptor Subtype-4 Agonist NNC 26-9100 Decreases Extracellular and Intracellular Aβ1-42 Trimers. Eur J Pharmacol 2012 May 15; 683 (1-3):116-24.
- Sandoval K.E., Farr S.A, Banks W.A., Niehoff M.L., Morley J.E., Crider A.M., and Witt K.A. Chronic peripheral administration of somatostatin receptor subtype-4 agonist NNC 26-9100 enhances learning and memory in SAMP8 mice. Eur J Pharmacol. 2011 Mar 1; 654 (1):53-9.
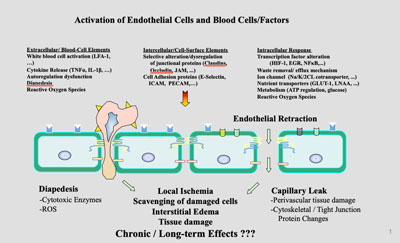 Sex, Diet, and inflammation impact Blood-brain barrier function
Sex, Diet, and inflammation impact Blood-brain barrier function
My team has researched alterations in the blood-brain barrier (BBB) under various conditions, including impact of high fat and low fat diets, fish-oil vs. lard. Other similar research has focused on the impact of peripheral LPS injections impact the BBB and changes associated steroids and how estrogens alter BBB tight junctional proteins.
- Sandoval K.E., Wooten J.S, Harris M.P., Schaller M.L, Umbaugh D.S., Witt K.A.
Mfsd2a and Glut1 Brain Nutrient Transporters Expression Increase with 32-Week Low and High Lard vs. Fish-Oil Dietary Treatment in C57Bl/6 Mice. Current Developments in Nutrition. July, 2018. - Witt K.A. and Sandoval K.E. Steroids and the blood-brain barrier: Therapeutic Implications. Pharmacology of the Blood Brain Barrier: Targeting CNS disorders. Adv Pharmacol. 2014;71:361-90.).
- Sandoval K.E. and Witt K.A. Age and 17β-estradiol effects on blood-brain barrier tight junction and estrogen receptor proteins in ovariectomized rats. Microvasc Res. 2011 Mar;81(2):198-205.
- Huber J.D., Witt K.A., Hom S., Egleton R.D., Mark K.S., and Davis T.P. Peripheral Inflammatory Pain Induces Blood-Brain Barrier Tight Junction Reorganization. Am. J. Physiol. Heart Circ. Physiol. 2001 Mar; 280 (3):H1241-248.
Stroke, hypoxia, and reoxygenation impact on the blood-brain barrier
Ischemic stroke, in conjunction with hypoxia, reperfusion, and reoxygenation are significant contributors to BBB alteration and negative outcomes respective to tight junctional permeability. Understanding how the BBB is altered in such states allows for optimizing timing of therapies as well as targeting options for treatments.
- Sandoval K.E. and Witt K.A. Blood-Brain Barrier Tight Junction Permeability and Ischemic Stroke Neurobiology of Disease, 2008, Nov; 32(2):200-19.
- Witt K.A., Mark K.S., Sandoval K.E., and Davis T.P. Reoxygenation stress on blood-brain barrier paracellular permeability and edema in the rat. Microvascular Research, 2008 Jan;75(1):91-6.
- Witt K.A., Mark K.S., Huber J.D., and Davis T.P. Hypoxia Inducible Factor and Nuclear Factor Kappa-B Activation in Blood-Brain Barrier Endothelium Under Hypoxic/Reoxygenation Stress J. Neurochem., 2005 Jan;92(1):203-14.
- Witt K.A., Mark K.S., Hom S, and Davis T.P. Effect of hypoxia/reoxygenation on rat blood-brain barrier permeability and tight junctional protein expression. Am. J. Physiol. Heart Circ. Physiol.. 2003 Dec;285(6):H2820-31.
Drug delivery of opioid peptides and pain
Since my early graduate research I have been involved in drug delivery, with primary emphasis on brain delivery. This research has also focused on opioid peptides targeting pain relief via delta, kappa, and mu receptors. The impact of these projects has contributed both to the further understanding of opioid pain pathways and the advancement of new opioid peptide-based compounds.
- Huber J.D., Campos C.R., Egleton R.D., Witt K.A., Guo L., Roberts M.J., Bentley M.D., Davis T.P. Conjugation of low molecular weight poly(ethylene glycol) to biphalin enhances antinociceptive profile. J Pharm Sci. 2003 Jul;92(7):1377-85.
- Witt K.A., Huber J.D., Egleton R.D., Roberts M.J., Bentley M.D., Guo L., Wei H., Yamamura H.I., and Davis T.P. Pharmacodynamic and Pharmacokinetic characterization of poly(ethylene glycol) conjugation to met-enkephalin analog [D-Pen2, D-Pen5]-enkephalin (DPDPE). J. Pharm. Exp. Ther. 2001 Aug; 298 (2):848-56.
- Witt K.A., Huber J.D., Egleton R. D., and Davis T.P. Insulin Enhancement of Opioid Peptide Transport Across the Blood-Brain Barrier and Assessment of Analgesic Effect. J. Pharm. Exp. Ther. 2000 Dec; 295(3):972-978.
- Witt K.A., Slate C.A., Egleton R.D., Huber J.D., Yamamura H.I., Hruby V.J., and Davis T.P. Assessment of Stereoselectivity of Trimethylphenylalanine Analogs of d-Opioid [D-Pen, D-Pen2,5] Enkephalin. J. Neurochem. 2000 Jul; 75(1











