
Michael Crider, PhD
Chair, Professor of Pharmaceutical Sciences
Associate Dean for Research
Phone: 618-650-5162
Fax: 618-650-5145
E-mail: [email protected]
Medicinal Chemistry
B.S., 1970, Murray State University
Ph.D., 1975, University of Kentucky
Postdoctoral, 1975-1977, Purdue University
Biography
Dr. Mike Crider is chair of the Department of Pharmaceutical Sciences, professor and associate dean of research. He earned the BS degree in chemistry from Murray State University and the PhD degree in medicinal chemistry from the University of Kentucky. Dr. Crider did postdoctoral work in the Department of Medicinal Chemistry at Purdue University, followed by faculty appointments at the University of Toledo and the University of Louisiana at Monroe. Dr. Crider’s research is focused on the discovery of compounds that affect the central nervous system.
Research
Over the course of my academic career, my research has been focused on the discovery of novel compounds with potential therapeutic effects in the central nervous system, prodrugs, and peptidomimetics. During my graduate work at the University of Kentucky, I was involved in the design of compounds with potential long-acting anticonvulsant effects. My graduate work stimulated an interest on the chemistry of five-membered nitrogen heterocycles throughout my academic career. We continue to pursue the discovery of novel anticonvulsants with new modes of action.
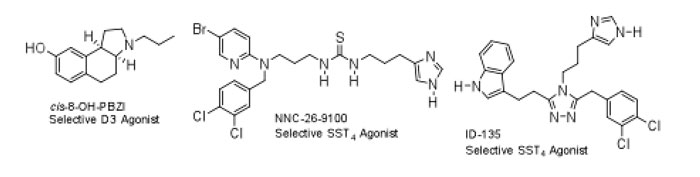
My interest in dopaminergic ligands stems from my postdoctoral work at Purdue University under the direction of Professor John Cassady. At the time, we were interested in the development of prolactin inhibitors based on the ergoline ring system. Dopamine was known to be an inhibitor of prolactin release and that certain breast tumors were prolactin-dependent. Thus, the discovery of dopaminergic ligands having prolactin inhibiting activity was of potential therapeutic value. After leaving Purdue for my first faculty position at the University of Toledo, I became involved in the synthesis of ergoline fragments having dopaminergic activity. However, the piperidine ring (D-ring) contained in the ergoline ring system was replaced by the five-membered pyrrolidine ring system. This research was pursued in collaboration with Peter Andersen and Mark Scheidelar at Novo Nordisk A/S in Denmark. We discovered that the tricyclic benz[e]indole ring system provided the framework for compounds with potent affinity for the dopamine D3 receptor. In collaboration with Dr. Eldo Kuzhikandathil at Rutgers University, we recently utilized the cis and trans-isomers of the benz[e]indole, 8-OH-PBZI, for homology modeling and determining the role of the D3 receptor tolerance and slow response termination (SRT) in signaling and behavior.
Another major area of research in my laboratory over the last 20 years has been in the development of nonpeptide ligands of somatostatin [somatotropin release-inhibiting factor (SRIF)]. Research from a number of laboratories has shown that the β-turn, Phe 7-Trp 8-Lys 9-Thr 10, is important for biological activity with residues Trp 8 and Lys 9 being essential. Additional studies have shown that the Phe 6 and Phe 11 residues may help stabilize the bioactive conformation of SRIF. SRIF exerts its biological effects by binding to a family of G-protein-coupled receptors (SST1-SST 5). Due to its poor bioavailablity and rapid degradation by proteases, the therapeutic utility of SRIF-14 is limited. As a result, the development of peptidomimetics of SRIF is of considerable interest. A broad screening program was initiated with collaborators at Novo Nordisk A/S focusing on a scaffold to which Phe 7, Trp 8, and Lys 9 mimetics were attached. This focused screen led to the discovery of a thiourea (NNC 26-9100) with high affinity (K i = 6 nm) for the subtype 4 somatostatin receptor (SST4). This was the first report of a nonpeptide exhibiting high binding affinity and selectivity at a cloned human receptor. Since the 1980s, several studies have shown that SRIF levels are reduced in the hippocampus and brain cortex in Alzheimer’s disease (AD) patients. Research has also shown that the enzyme neprilysin, an Aβ-peptidase, regulates the steady state levels of Aβ1-42 and Aβ1-40. Furthermore, SRIF has been shown to enhance neprilysin activity. With reduced levels of SRIF, neprilysin activity declines, leading to higher levels of Aβ and toxicity. In collaboration with Drs. Ken Witt and Karin Sandoval, we have reported that acute administration of the SST4 agonist NNC-26-9100 increases activity of neprilysin, leading to lower levels of Aβ and the trimeric Aβ oligomer within extracellular and intracellular fractions. Thus, sst4 agonists may have potential benefit in the treatment of AD. Recent studies have led to the development of the 1,2,4-triazole (ID-135), a high affinity and selective SST4 agonist.
Selected Publications
Kota, K.; Kuzhikandathil, E.V.; Afrasiabi, M.; Lacy, B.; Kontoyianni, M.; Crider, A.M.; Song, D. Identification of key residues involved in the activation and signaling properties of dopamine D3 receptor. J. Pharmacol. Res. 2015, 99, 174-184.
Omran, A.; Eslamimehr, S.; Crider, A.M.; Neumann, W.L. Synthesis of 3-(3-hydroxyphenyl)pyrrolidine dopamine D3 receptor ligands with extended functionality for probing the secondary binding pocket. Bioorg. Med. Chem. Lett. 2018, 28, 1897-1902.
Daryaei, I.; Sandoval, K.; Witt, K.A.; Kontoyianni, M.; Crider, A.M. Discovery of a 3,4,5-Trisubstituted-1,2,4-Triazole Agonist with High Affinity and Selectivity at the Somatostatin Subtype-4 (sst4) Receptor. Med. Chem. Commun. 2018, 9, 2083-2090.
Currier, V.; Molki, M.; Fryman, K.; Rodgers, L.D.; Crider, A.M. Synthesis and Anticonvulsant Activity of α-Amino Acid Amide Derivatives. Curr. Bioact. Compd. 2019, 15, 547-561.
Neumann, W.L.; Sandoval, K.E.; Mobayen, S.; Minaeian, M.; Kukielski, S.G.; Srabony, K.N.; Frare, R.; Slater, O.; Farr, S.A; Niehoff, M.L.; Hospital, A.; Kontoyianni, M.; Crider, A.M.; Witt, K.A. Synthesis and structure-activity relationships of 3,4,5-trisubstituted-1,2,4-triazoles: high affinity and selective somatostatin receptor-4-agonists for Alzheimer’s disease treatment. RSC Med. Chem. 2021, 12, 1352-1365.
3,4,5-Trisubstituted-3-Thio-1,2,4-Triazoles and 3,4,5-Trisubstituted-3-Thio-1,2,4-Triazoles and Uses Thereof. Witt, K.A.; Crider, A.M.; Neumann, W.; Hospital, A.; Sandoval, K.; Kontoyianni, M. US Patent, 11,136,312 B2, October 5, 2021










